Removing the biggest bottleneck in inventing life-changing treatments
My chat with Brandon Li, Cofounder of Power
Today’s Scatter Brain is brought to you by AngelList!
AngelList Stack is for startups that want faster fundraising, cleaner cap tables, and high-interest banking all in one place. Scaling companies such as Abound, Harness Wealth and Syndicate migrated from other vendors in less than a week with zero legal fees to gain an unfair advantage in managing their back-office. Learn more by signing up here.
Clinical trials are a critical step in making progress in medical treatments. I have wondered how we managed to build the covid vaccines at a breakneck speed and why can’t we replicate that success across treatments. It is easy to think that speeding up the trials would cause a positive change in the medical progress we make. A big part of that is timely and appropriate enrollment and participation in trials. When I saw Footwork’s Nikhil’s latest investment announcement, I reached out to Power’s Cofounder, Brandon, to talk about how he wants to make clinical trials accessible to anyone, regardless of background, location, or network.
In this chat, Brandon and I talked about :
The current state of things in the clinical trials world
Digitization journey of clinical trials
Opportunity for a patient-centric marketplace
What the critics get wrong
Early interactions with the product
Building a heterogenous marketplace
Value for the patients, researchers & sponsors
Managing liquidity
Supply segmentation by type of treatment
Balancing startup DNA with industry knowledge
Sar: Most people don’t spend much time thinking about clinical trials. And that’s a good thing! It is a very serious matter for those who have to go down that path for life-changing medical care. What minimum viable context would outsiders need to understand the clinical trials world? I think there are a few basic things that people can probably intuit from basic reading: Clinical trials have micro benefits for patients and macro benefits for society in terms of medical progress; we would all be better off by speeding up the matching process between trials and patients, and that clinical trial participation is an ongoing ethical debate.
Brandon: The minimum viable context is interesting framing. Here’s how I’d put it:
Self-directed research for trials has grown 22x in the last 7 years. This is consistent with a broader movement towards taking control of your health – patients are increasingly doing their research – we call them the Empowered Patient. All roads lead to clinicaltrials.gov. Anyone who’s sent a patient there knows it will suck.
Roughly 86% of trials are delayed, and plenty are put on pause indefinitely. Patient recruitment is the biggest bottleneck in life science and one of the primary reasons new treatments can take 10 years to get to patients.
COVID set a new floor on trial speed. All the experts said it would take at least 7 yrs to get a vaccine. It took <2 yrs! Two major things were different: First, the trials were designed to be rolling/parallel versus sequential. Second, there was no waiting around for patients to participate. The trial design is a function of regulatory acceptance and the availability of patients to participate.
Most companies prioritise pharma needs, which has created a gap in the market (our opportunity) to build the first consumer-centric, horizontal platform for patients.
Sar: Is the clinical trials world in the OpenTable phase of its digitization journey?
Brandon: We are currently in the listings phase and entering the OpenTable phase. Today, clinical trial information either exists on clinicaltrials.gov (horizontal, non-patient friendly) or individual pharma, CRO, and hospital websites (vertical, somewhat patient friendly). The former doesn’t work because you can’t connect with researchers. The latter doesn’t work because patients don’t want to go and read 5000 different websites.
The consumer experience usually ends with a phone number to call (scary!) or a call center reaching out (salesy). We’re in a time when a) consumers want to do more transacting in a self-serve way online, and b) trials are tired of chasing down every patient over the phone in a prolonged outbound sales motion.
It’s time for a true marketplace experience.
Sar: What were the naysayers saying when you got started? What did they get wrong?
Brandon: People were (rightly, I think) concerned about the graveyard of prior attempts in this space. At first glance, it’s easy to see many past attempts and wonder whether a company could achieve a different outcome.
I think they missed that no one has truly built a patient-first experience. This is a notably unique take. The second-order effect is organic growth. Patients and physicians see a viable alternative to clinicaltrials.gov and choose to come to Power. Others have had to grow via paid acquisition without a patient-centric approach. This is a hard way to grow a marketplace with necessarily slow monetization loops in the beginning.
Sar: What early ideas were wrong?
Brandon: We first wanted to build an “Uber for clinical trials.” We thought we could build a giant database of patients, let researchers request patients, do the matching for them, and then have patients show up. Unfortunately, this was wrong for three reasons:
Patient intent expires over time: We thought researchers would request patients, and then patients would show up to participate. But, patients are typically not interested in trials six months after they sign up. They have probably already started down a different treatment path. The pool of sign-ups you’d accumulate becomes stale quickly, and the on-demand requests don’t work as a result.
Build for patients, not researchers: Our first instinct, like everyone else’s, was to build for the researchers. Remember: 86% of trials are delayed! They have a huge problem to solve. But once we realized how patient intent expires, we knew we needed to build a product for patients while they were searching.
This is a heterogeneous, not homogeneous marketplace: In a homogeneous marketplace like Uber, users don’t care which rider/driver they are paired with. As a result, you can build a platform-driven match experience. Demand (patients) sees supply (trials) as fairly unique. Patients care deeply about which trial they get – we needed to build a user-driven match experience.
Sar: What parts of the clinical trial process does Power play a role in today, and do you have plans to go after the remaining parts?
Brandon: Today, Power operates in patient recruitment: we help motivated patients find and get access to leading medical researchers. This in and of itself is a massive undertaking. But who knows what the future has in store ;)
Sar: How did you go about acquiring the supply side?
Brandon: To focus on the patient experience, we began by seeding the supply side with publicly available information. Luckily for us, there are plenty of publicly available details about clinical trials. This allowed us to build a rich demand-side experience without needing to get live supply on the marketplace. We’re currently in closed beta with a handful of supply-side partners to ensure they can onboard and use the platform with minimum friction.
Sar: Have you had pushback from researchers on getting listed without their consent?
Brandon: There will always be a spectrum of early adopters and those skeptical of change. Largely, researchers have loved our product: they resonate deeply with the need for a consumer-friendly alternative to clinicaltrials.gov. Most will say, “I’ve always thought someone should do this.” For the naysayers: at the end of the day, we’re building on top of publicly available information. If they don’t like it, they can email me, and we can update their profiles.
Sar: Besides researchers, you also help the sponsors. Can you talk about what those two groups do and how Power serves each of them?
Brandon: Researchers are on the ground in the clinic, working daily with patients (doctors and nurses that provide treatment to the patients). There are usually multiple research teams for each trial – each hospital or location has a team of researchers. We think of each unit of supply as a trial x site combination. We enable research teams to connect with patients directly.
Sponsors own the IP of the potential treatments (these are the life science companies). They are responsible for the end-to-end trial. Sponsors will partner with researchers across many hospital sites to administer patient treatments. They are thinking about the trial progress but have no day-to-day interactions with patients. We deliver faster trial recruitment timelines across multiple sites.
Sar: How did you find your first 100 patients?
Brandon: Kickstarting a marketplace is always hard. We did a bunch of things we knew were not long-term solutions. We ran some paid ads to prove that patients would find the platform useful. We had no intention of this being a sustainable growth channel, but it was the fastest way to understand if/how patients would use Power when they found us
Patients are fairly active in self-organizing communities. We joined a bunch of Facebook groups and Reddit boards to post links to our website and see if people would click. Eventually, people started sharing links to us organically, growing word-of-mouth traffic.
Sar: What resonates with the patients the most today?
Brandon: We operate in a space where the status quo is intensely painful (i.e., clinicaltrials.gov). We make it easy to do simple things like searching for their nearest location. That sounds like table stakes but is impossible to do on the gov website.
Patients love that they no longer need to tab between multiple clinical trial websites. They only ever need to come to Power for clinical trial options. Patients can contact trials directly on-platform. It doesn’t start with a call.
Sar: You have a narrow time window when patients need to enroll for trials, and the trials need more patients. How do you think about creating value in that window?
Brandon: Distribution is an advantage on the demand side when patient intent expires. We want to be where patients are when demand appears at first: on Google, in advocacy groups, word of mouth among patients and physicians. Our patient-centric product philosophy does a lot of the heavy lifting here. It’s easy to refer a delightful product.
Sar: Does that mean you have to constantly search for new users because of the one-time engagement of your offering amongst most of the patients? What can Power do for those that have already signed up?
Brandon: We have to constantly earn the right to be the patient’s go-to destination for clinical trials. This forces us to remain focused on our north star of patients. I also think that the distribution advantage is compounding. Look at Zillow, for example – each new home-buying moment is a new acquisition. They’ve built advantages in the brand and organic search, which are defensible. As patients create profiles, we learn more about them and can tee up recommendations they haven’t considered yet. We can also place them on a waitlist and reach out when a new trial is started that matches their needs.
Sar: The vast majority of at-scale consumer marketplaces have a high degree of fungibility on at least one side. You mentioned one of your early learnings was that Power is a heterogenous marketplace. Airbnb hosts don’t quite care about who the guests are as long as they meet some basic expectations. Riders don’t care about who their Uber drivers are. You do not have that dynamic on either side. First, is that a correct read? Second, can you explain how geography and medical conditions affect supply and demand segmentation?
Brandon: I don’t agree with fungibility being a requirement of at-scale marketplaces. Long tail matching problems are where marketplaces create a ton of value because supply/demand isn’t commoditizable: Craigslist is still around because there’s such a long tail of supply and demand to match.
For the supply side, demand is fungible as long as patients are qualified to participate. For the demand side, patients want to feel comfortable knowing they’ve selected the best trial for themselves. We need to allow them to browse, filter, compare, etc.
The patient side differs by condition/category. Less severe conditions are more local. Patients with more severe conditions are willing to travel further. On the supply side, it differs by stage of the trial. The earlier the research, the more likely it is to be contained in one geography. But later stage and larger trials are inherently national/international. We already have trials asking us if we support multiple countries.
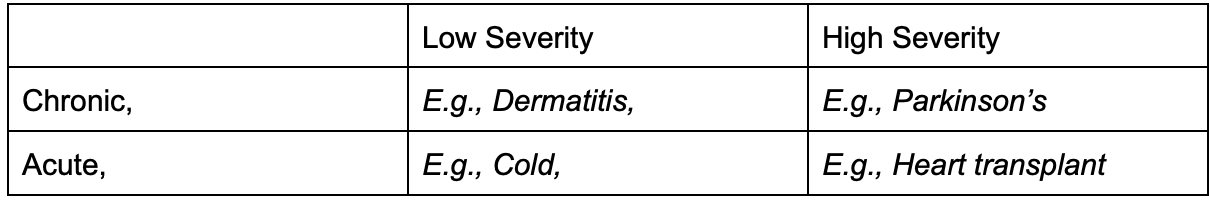
Sar: It sounds like you could draw a two-by-two matrix of your supply.
Brandon: Ha – we do have a 2x2 on supply. Roughly the axes we think about are severity and whether a condition is chronic or acute. High severity + chronic conditions have the most engaged patients. Low severity + chronic conditions have moderately engaged patients who might participate in multiple studies over many years. Low severity + acute conditions usually resolve themselves. And high severity + acute conditions are usually resolved without patient involvement (e.g., a surgeon makes a call on the operating table).
Sar: I’m curious how you think about creating patient trust and comfort. Do you think there is room for Power to play a role in helping patients decide which trials are best for them?
Brandon: Power builds trust by providing the most comprehensive information transparently, and simply. We will never become the go-to destination for patients if they feel the need to cross-reference us against other resources. People can tell when they’re being sold something on the internet. We need to give consumers more credit. If you look at the content out there, it’s uncomfortably salesy. Most people don’t have the time or technical expertise to dig into the PhD-level content.
Sar: How do you think about balancing leaning in on your startup DNA and avoiding blindspots that industry insiders might not have?
Brandon: I think unfair advantages need to be part of the founding DNA. Capabilities that are common in the industry can be brought in. Many people in clinical trials know about clinical trial operations – we can bring that in. We’re not building a biotech company where the core research is our unfair advantage. So our approach is to surround ourselves with experts in pharma via investors and advisors. We have Jeff Kindler, the former CEO of Pfizer, as an investor, along with a few other industry leaders who help us get smart on the intricacies of trial operations, ethics, and more.
Sar: What’s on the horizon for Power?
Brandon: We’ve 10x’d YTD.
We will continue differentiating on the patient experience. While we think we’ve already got a 10x product versus the status quo, we’re not satisfied. We need to earn the right to be the go-to destination every day. We are in closed beta for Power for research teams. So far, the feedback has been tremendous, but I won’t let the cat out of the bag just yet.
Over the last few months, we’ve had lots of inbound interest from the life science industry. We’ve tried to be measured in who we onboard, but our patient-facing product is now at a scale where we can meaningfully support a lot of these asks.
AngelList Stack is for startups that want faster fundraising, cleaner cap tables, and high-interest banking all in one place. Learn more by signing up here.
Recent chats :
Accents as a sign of bravery with Anada Lakra, CEO of BoldVoice
Turning college savings into a team sport with Jordan Lee, CEO at Backer
Reflections on being a CEO and building a real estate marketplace, with Anthemos Georgiades, CEO of Zumper
Simplifying tax filing experience with Gavin Nachbar, CEO of Column Tax
Moving money without freaking out the CFO with Daniel Yubi, CEO of Payable
Building smarter pipes for debt with Danielle Pensack, Cofounder of Rightfoot